Background
The gene upstream binding transcription factor ( UBTF ) encodesa nucleolar protein that is a key regulator of the epigenetic status of ribosomal DNA and RNA transcription. Tandem duplications (TDs) of the UBTF gene have been initially identified in pediatric patients (pts) with acute myeloid leukemia (AML) and were recently also reported as relatively rare, but recurrent genetic events in adult AML based on targeted re-sequencing efforts (1.2%-3%). Their presence was associated with dysplastic bone marrow features, and co-occurrence of select genetic features, including FLT3-internal tandem duplications (ITDs) and WT1 mutations, and inferior outcome. However, associated biologic features and affected pathways have not yet been thoroughly investigated, and may provide further insights into leukemogenesis and identify potentially targetable vulnerabilities. Thus, we set out to assess the frequency of UBTF-TDs in a large cohort of adult AML pts (n=807) treated on frontline protocols of the Alliance for Clinical Trials in Oncology with complete RNA sequencing data, comprehensive targeted mutation profiling, centrally reviewed cytogenetic data and clinical information available, and to gain further insights into UBTF-TDs via comprehensive integrated genomic profiling.
Methods
UBTF-TD detection was performed via the RNAseq-based RNAmut and EnFusion algorithm. To gain insights into associated molecular features, all UBTF-TD + pts underwent paired tumor-normal whole-exome sequencing. To understand associated pathways and downstream effects, differential gene expression (DGE) and gene set enrichment analyses (GSEA) were performed.
Results
UBTF-TDs were detected in 6/807 pts (0.7%). In line with previous studies, these 6 UBTF-TD + pts were young, with a median age of 35y (range: 19-59y). Three pts had extramedullary disease, 2 with skin and 1 with gingival involvement. All but 1 of the 6 pts were cytogenetically normal. Clinically, pts did poorly after treatment with intensive chemotherapy induction, with a CR rate of only 33%. No pt was alive 2.5 y after diagnosis.
In paired tumor/normal exome sequencing, UBTF-TD + pts had a median mutation burden of 21 non-synonymous variants in coding genes (range: 17-27), with a notable presence of larger insertions/deletions (indels), loss of heterozygosity (LOH) or TDs as co-occurring somatic genetic lesions (median: 4, range: 2-5). There was a 100% co-occurrence of UBTF-TDs with FLT3-ITDs. In addition, pts harbored larger indels in WT1 (n=2), BPTF, CEBPA, Corf53, KRTAP5-7, NFE2, PTPRN2, RNF144B, SOWAHC and ZCCHC14. Also, analysis of copy-number variations and LOH revealed LOH in 5 pts, in chromosomes 13 (n=3), 15 (n=1) and 18 (n=1), suggestive of a propensity to the acquisition of structural variants.
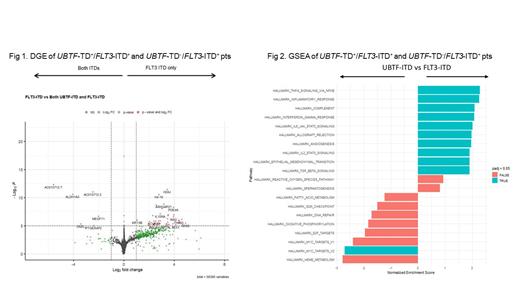
In unsupervised clustering analysis of the RNAseq data, UBTF-TD + pts clustered with FLT3-ITD + pts but formed a relatively distinct subcluster in principal component and t-distributed stochastic neighbor embedding analyses. In supervised clustering comparing 6 UBTF-TD +/ FLT3-ITD + and 196 UBTF-TD -/ FLT3-ITD + pts, 2 UBTF-TD - pts clustered together with the UBTF-TD +/ FLT3-ITD + group, suggesting either presence of an undetected TD or genetic phenocopy. UBTF expression was higher in UBTF-TD pts. DGE analysis of UBTF-TD +/ FLT3-ITD + and UBTF-TD -/ FLT3-ITD + pts (Fig 1) showed distinct upregulation of the locus of the stem cell and chemoresistance marker ALDH1A3, including the associated long non-coding RNAs AC015712.7/AC015712.2 in UBTF-TD + pts, which was confirmed by a comparison of those pts to AML pts who had neither TD (n=605). GSEA indicated a downregulation of inflammation pathways and several metabolic pathways of UBTF-TD +/ FLT3-ITD + compared to FLT3-ITD + pts (Fig 2).
Conclusions
UBTF-TDs are rare, but recurrent genetic events in adult AML, associated with high chemotherapy resistance. Our results highlight a susceptibility of those pts to the acquisition of structural variants and suggest a distinct associated biology, with upregulation of ALDH1A3 as possible contributing resistance mechanism.
Support: U10CA180821, U10CA180882, U24CA196171; Clinicaltrials.gov Identifiers: NCT00048958 (CALGB 8461), NCT00899223 (CALGB 9665), NCT00900224 (CALGB 20202); https://acknowledgments.alliancefound.org
Disclosures
Curran:Incyte: Other: Advisory board; Amgen: Other: Advisory board; Servier: Consultancy, Other: Expert consensus panel; Kite: Other: Advisory board; Pfizer: Honoraria, Other: Advisory board; Jazz: Other: Advisory board. Walker:Karyopharm Therapeutics Inc.: Consultancy, Current Employment, Current equity holder in publicly-traded company. Mims:Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Uy:Jazz: Other: Advisory Board. Stock:Servier: Other: Data Safety Monitoring Board/Advisory Board; Kura: Research Funding; Kite: Consultancy; Jazz Pharmaceuticals: Consultancy, Honoraria; Glaxo Smith Kline: Consultancy; Amgen: Honoraria; Newave: Honoraria. Stone:Syntrix: Other: DSMB; Rigel: Consultancy; Kura One: Consultancy; Jazz: Consultancy; Cellularity: Consultancy; BerGenBio: Consultancy; AvenCell: Consultancy; Amgen: Consultancy; Takeda: Other: DSMB; Lava Therapeutics: Consultancy; Ligand Pharma: Consultancy; Hermavant: Consultancy; GSK: Consultancy; Epizyme: Other: DSMB; Aptevo: Other: DSMB; CTI Biopharma: Consultancy; Abbvie: Consultancy. Byrd:Newave: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kurome: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Vincerx: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; OSU Drug Devel. Inst.: Consultancy; Orbimed: Consultancy, Research Funding; Eilean Therapeutics: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Orange Grove Bio: Membership on an entity's Board of Directors or advisory committees; American Cancer: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Other: TRAVEL, ACCOMMODATIONS, EXPENSES. Eisfeld:Karyopharm Therapeutics: Other: spouse employment; Astra Zeneca: Honoraria, Other: CEI Advisory Board; OncLive: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal